Human Health Division (HHD) Products
Product List by Brand Name
Agonil Cream

Agonil Cream

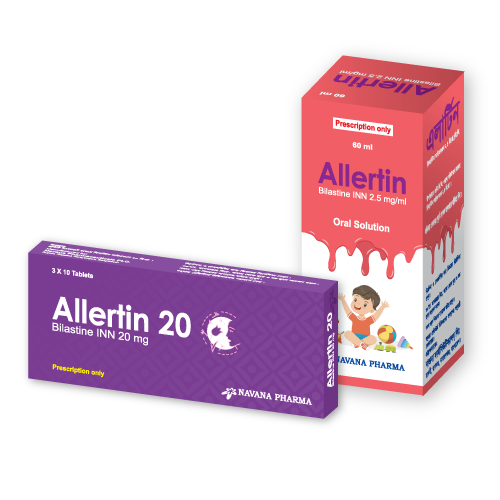
Allertin
Aloglip

Azirox

Composition:
Azirox™ 500 Tablet: Each film coated tablet contains Azithromycin Dihydrate USP equivalent to Azithromycin 500 mg. Azirox™ Powder for Suspension (20 ml, 35 ml & 50 ml): Each 5 ml reconstituted suspension contains Azithromycin Dihydrate USP equivalent to Azithromycin 200 mg.
Indication:
Acute bacterial exacerbations of chronic obstructive pulmonary disease, Community-acquired pneumonia, Acute otitis media, Sinusitis, Pharyngitis, Tonsillitis, Skin and soft tissue infections, Urethritis and Cervicitis, Genital ulcer disease, Sexually transmitted diseases (STD), Typhoid fever.
Dosage and Administration:
Adult dose: For Acute bacterial exacerbations of chronic obstructive pulmonary disease, community-acquired pneumonia, sinusitis, pharyngitis, tonsillitis and Skin and Soft tissue infections due to the indicated organisms: 500 mg once daily orally for 3 days or as an alternative given over 5 days with 500 mg dose on the first day followed by 250 mg once daily on days 2 through 5. Genital ulcer disease, non-gonococcal urethritis and cervicitis: 1 g (1000 mg) as a single dose. Urethritis and cervicitis due to Neisseria Gonorrhoeae: 2g (2000 mg) as a single dose. Sexually transmitted diseases: 1 g as a single dose. Typhoid fever: 500 mg once daily for 7 days. Pediatric dose: For children over 6 months recommended dose is 10mg/kg once daily for 3 days 15-25 kg: 200 mg once daily for 3 days, 26-35 kg: 300 mg once daily for 3 days, 36-45 kg : 400 mg once daily for 3 days, Azirox™ should be taken at least 1 hour before or 2 hour after meal or as directed by the physician.
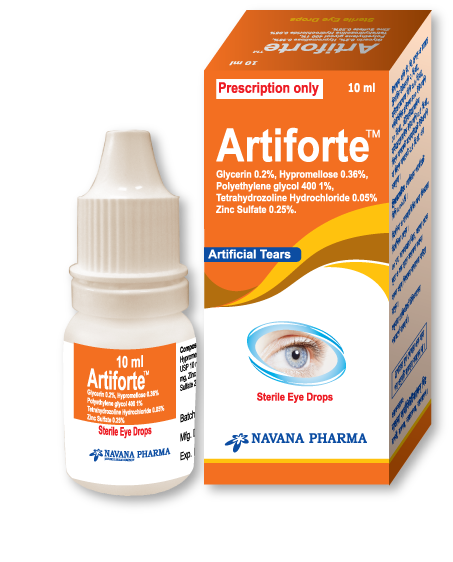
Artiforte
Arokast

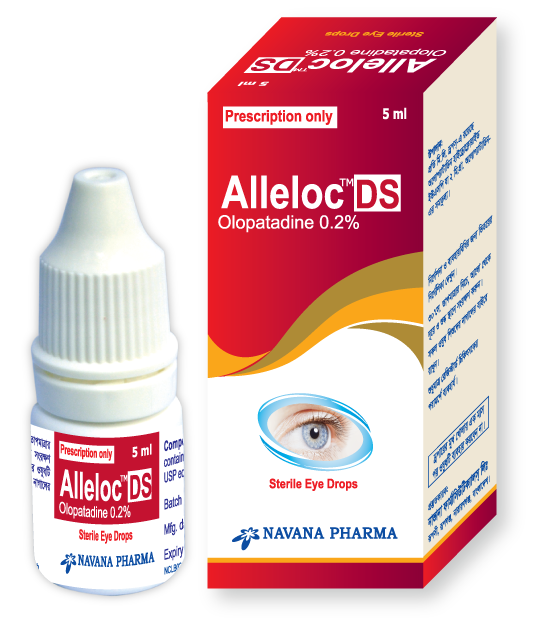
Alleloc DS