Faropenem
Composition
Faropen 150 Tablet: Each film coated tablet contains Faropenem Sodium INN equivalent to Faropenem 150 mg.
Faropen 200 Tablet: Each film coated tablet contains Faropenem Sodium INN equivalent to Faropenem 200 mg.
Indications
Faropenem is indicated for treatment of the following infections:
• Urinary tract infections: Pyelonephritis, cystitis, prostatitis, seminal
gland inflammation.
• Lower respiratory tract infections: Acute bronchitis, pneumonia,
pulmonary suppuration.
• Ear, nose and throat (ENT) infections: Otitis externa, tympanitis, sinusitis
• Upper respiratory tract infections: Pharyngitis, tonsillitis.
• Skin and skin structure infections: Pustular acne, folliculitis,
contagious impetigo, erysipelas, lymphangitis, suppurative nail inflammation, subcutaneous abscess, hidradenitis (sweat gland inflammation), infective sebaceous cyst, chronic pyoderma, secondary infection of external wounds or surgical wound.
Dosage and Administration
150 or 200 mg should be taken three times daily for 7 to 14 days.
Spinosad
Composition:
Freescab Topical Suspension: Each ml of topical suspension contains Spinosad INN 9 mg.
Indication:
Scabies Infestations: Spinosad Topical Suspension is a scabicide indicated for the topical treatment of scabies infestatons in adult and paediatric patients 4 years of age and older.
Head Lice Infestations: Spinosad Topical Suspension is a pediculicide indicated for the topical treatment of head lice infestations in adult and paediatric patients 6 months of age and older.
Dosage & Administration:
For topical use only. Not for oral, ophthalmic, or intravaginal use.
Pimecrolimus INN 1%
Composition
Pmec Cream: Each gram cream contains Pimecrolimus INN 10 mg.
Indication
Pmec Cream is indicated as second-line therapy for the short-term and
noncontinuous chronic treatment of mild to moderate atopic dermatitis in
non-immunocompromised adults and children 2 years of age and older, who have failed to respond adequately to other topical prescription treatments or when those treatments are not advisable.
Dose & Administration
Skin should be cleaned before applying the cream. One finger tip unit (measured by distance from the tip of an adult index finger to the first crease of that finger) to be applied thinly and evenly on the affected area of skin twice daily. (PHYSICIAN
MAY ADJUST THE DOSE). Treatment should be stopped if signs and symptoms
(e.g. itch, rash and redness) resolve. If signs and symptoms persist and beyond 6
weeks, patients should be re-examined to confirm the diagnosis of atopic
dermatitis. A patient should not use sun lamps, tanning beds, or get treatment with
ultraviolet light therapy during treatment with Pmec Cream. Continuous long-term
use of Pimecrolimus should be avoided. A patient should not cover the skin being
treated with bandages, dressings or wraps. Loose fitting clothing that protects the
treated area from the sun are encouraged.
Econazole Nitrate + Triamcinolone Acetonide
Composition:
Stenide Cream: Each gram cream contains Econazole Nitrate BP 10 mg and Triamcinolone Acetonide BP 1 mg.
Indication:
For the topical treatment of inflammatory dermatomycoses and
inflammatory skin conditions which can be complicated by or threatened by bacterial or fungal skin infection.
Dosage & Administration:
Apply by gently rubbing onto the skin twice daily for 14 days or, as
directed by the physician.
Diethylamine Salicylate
Composition:
Agonil Cream: Each gram cream contains Diethylamine
Salicylate BP 100 mg.
Indications:
For symptomatic relief of rheumatic and minor
musculo-skeletal conditions including lumbago, fibrositis,
sciatica, bruises and strains.
Dosage & Administration:
Apply three times daily to the affected area, massaging until cream is fully absorbed.
Diethylamine Salicylate
Composition:
Agonil Cream: Each gram cream contains Diethylamine
Salicylate BP 100 mg.
Indications: For symptomatic relief of rheumatic and minor musculo-skeletal conditions including lumbago, fibrositis, sciatica, bruises and strains.
Dosage & Administration:
Apply three times daily to the affected area, massaging until cream is fully absorbed.
Vonoprazan
Composition
Vontac 10: Each film coated tablet contains Vonoprazan fumarate INN equivalent
to Vonoprazan 10 mg.
Vontac 20: Each film coated tablet contains Vonoprazan fumarate INN equivalent
to Vonoprazan 20 mg.
Pharmacology
Vonoprazan is a novel potassium-competitive acid blocker (P-CAB) that works through a
reversible potassium-specific competitive mechanism to inhibit active proton pumps
directly. Vonoprazan does not require acid activation or any time constraints for its
administration. As a result of its rapid onset and prolonged action, Vonoprazan is a
suitable option for PPI.
Vonoprazan can be taken regardless of meal ingestion and the rate of absorption is not
affected by meals. The absorption speed of Vonoprazan is rapid, and the time taken to
reach maximum concentration in plasma is less than 2 hours after oral administration.
After absorption, the T1/2 in plasma is approximately 2 hours for conventional PPIs, but
up to 9 hours for vonoprazan. Therefore, Vonoprazan stays in the blood longer and can
block acid secretion continuously.
Indication
• Treatment of gastric ulcer, duodenal ulcer, reflux esophagitis; prevention of recurrent
gastric or duodenal ulcer associated with low-dose aspirin administration; and
prevention of recurrent gastric or duodenal ulcer associated with non-steroidal
anti-inflammatory drug administration
• Adjunct therapy to Helicobacter pylori eradication in the following: Gastric or duodenal
ulcer, Gastric Mucosa Associated Lymphoid Tissue (MALT) lymphoma, idiopathic
thrombocytopenic purpura, the stomach after endoscopic resection of early-stage gastric
cancer, or Helicobacter pylori gastritis.
Dosage & Administration
• Gastric ulcer & duodenal ulcer: The usual adult dosage is 20 mg once daily, 8 week
treatment for gastric ulcer and a 6-week treatment for duodenal ulcer.
• Reflux esophagitis: The usual adult dose is 20 mg once daily for a total of 4 weeks of
treatment. If that dosing proves insufficient, the administration should be extended, but
for no longer than 8 weeks of treatment.
• For the maintenance therapy of reflux esophagitis showing recurrence. The dose is 10
mg once daily. When the efficacy is inadequate, the dosage may be increased up to 20
mg once daily.
• Prevention of recurrence of gastric or duodenal ulcer during low-dose aspirin
administration: The usual adult dose is 10 mg once daily.
• Prevention of recurrence of gastric or duodenal ulcer during non-steroidal
anti-inflammatory drug (NSAID) administration: The usual adult dose is 10 mg once
daily.
• Adjunct to Helicobacter pylori eradication: For adults, the following three-drug regimen
should be administered orally at the same time twice daily for seven days: 20 mg dose
of Vonoprazan, 750 mg dose of amoxicillin hydrate and 200 mg dose of clarithromycin.
• If Helicobacter pylori eradication with a three-drug regimen comprising a proton pump
inhibitor, amoxicillin hydrate and clarithromycin has been unsuccessful, as an alternative
treatment, adults should be administered the following three drugs twice daily for seven
days: 20 mg of Vonoprazan, 750 mg of amoxicillin hydrate and 250 mg of Metronidazole
or as directed by the physicians.
Ciprofloxacin
Composition
Floxacin 500 mg Tablet: Each tablet contains Ciprofloxacin
Hydrochloride USP equivalent to 500 mg Ciprofloxacin.
Indications
Infectious diarrhoea, Urinary tract infection, Typhoid fever, Lower
respiratory tract infection, Acute sinusitis, Bone and joint infection,
Chronic bacterial prostatitis, Gonococcal infection, Inhalational
anthrax.
Dosage & Administration
500 mg twice daily for 7 to 14 days
Meropenem
Composition
InpenTM 500 mg IV injection: Each vial contains Meropenem USP 500 mg.
InpenTM 1 g IV injection: Each vial contains Meropenem USP 1 g.
Indications
InpenTM is indicated for treatment, in adults and children, of the following infections caused by single or multiple susceptible bacteria and as empiric therapy prior to the
identification of the causative organisms: Lower Respiratory Tract Infections, Urinary Tract Infections, including complicated infections, Intra-abdominal Infections, Gynaecological infections, including
postpartum infections, Skin and Skin Structure Infections, Meningitis, Septicaemia, Empiric treatment, including initial monotherapy, for presumed bacterial
infections in host-compromised, neutropenic patient.
Because of its broad spectrum or bactericidal activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria. InpenTM is effective for the treatment of
polymicrobial infections.
Dosage and Administration
ADULTS:
Usual dose: 500 mg to 1 g by intravenous administration every 8 hours depending on type end severity of Infection.
Exceptions: 1. Febrile episodes to neutropenic patients - the dose should be I g every 8 hours. 2. Meningitis- the dose should be 2 g every 8 hours.
As with other antibiotic, caution may be required in using Meropenem as monotherapy in critically iII patients with known or suspected Pseudomonas aeruginosa lower
respiratory tract erections. Regular sensitivity testing is recommended when treating Pseudomonas aeruginosa infections.
InpenTM should be given as an intravenous bolus injection over approximately 5 minutes or by intravenous infusion over approximately 15 to 30 minutes (see Method of
Administration).
ELDERLY
No dosage adjustment is required for the elderly with normal renal function or creatinine clearance values above 50 ml/min.
CHILDREN
For infants and children over 3 months and up to 12 years of age, the recommended intravenous dose is 10 to 40 mg/kg every 8 hours depending on type and severity of
infection, the known or suspected susceptibility of the pathogen(s) and the condition of the patient. In children over 50 kg weight, adult dosage should be used.
Methenamine Hippurate
Composition
Methebac Tablet: Each tablet contains Methenamine
Hippurate USP 1 g.
Indications
Methebac is indicated for prophylactic or suppressive
treatment of frequently recurring urinary tract infections
when long-term therapy is considered necessary. This
drug should only be used after eradication of the
infection by other appropriate antimicrobial agents.
Dosage & Administration
1 tablet (1.0 g) twice daily (morning and night) for adults
and paediatric patients over 12 years of age. 1/2 to 1
tablet (0.5 to 1.0 g) twice daily (morning and night) for
paediatric patients 6 to 12 years of age. Since the
antibacterial activity of Methebac is greater in acid urine,
restriction of alkalinizing foods and medications is
desirable or as directed by the physician.
Ezetimibe & Atorvastatin
Composition
Ezitor 10/10: Each film coated tablet contains Ezetimibe USP 10 mg and Atorvastatin calcium USP equivalent to Atorvastatin 10 mg.
Ezitor 10/20: Each film coated tablet contains Ezetimibe USP 10 mg and Atorvastatin calcium USP equivalent to Atorvastatin 20 mg.
Indication
• reduce elevated total-C, LDL-C, Apo B, TG, and non-HDL-C, and to increase HDL-C in patients with primary (heterozygous familial and non-familial) hyperlipidemia or mixed hyperlipidemia.
• reduce elevated total-C and LDL-C in patients with homozygous familial hypercholesterolemia (HoFH), as an adjunct to other lipid-lowering treatments.
Dosage and Administration
• Dosage range is 10/10 mg/day through 10/80 mg/day.
• Recommended starting dose is 10/10 mg/day or 10/20 mg/day.
• Recommended starting dose is 10/40 mg/day for patients requiring a greater than 55% reduction in LDL-C or as directed by the physician.
Roflumilast
Composition
Lumilast Cream: Each gram cream contains Roflumilast INN 3 mg.
Indication:
Lumilast cream is indicated for topical treatment of plaque psoriasis,
including intertriginous areas, in patients 6 years of age and older.
Dosage & Administration:
Lumilast cream should be applied on affected areas once daily and rubbed in completely. Hands should be washed after application.
Lumilast cream is for topical use only and not for ophthalmic, oral, or intravaginal use or as directed by the physician.
Olmesartan + Amlodipine
Composition
Olmepin™ 20/5 Tablet: Each film coated tablet contains Olmesartan Medoxomil USP 20 mg & Amlodipine Besilate BP equivalent to Amlodipine 5 mg.
Olmepin™ 40/5 Tablet: Each film coated tablet contains Olmesartan Medoxomil USP 40 mg & Amlodipine Besilate BP equivalent to Amlodipine 5 mg.
Indication
Olmepin™ is indicated for the treatment of hypertension, alone or with other antihypertensive agents.
Olmepin™ may also be used as initial therapy in patients who are likely to need multiple antihypertensive agents to achieve their blood pressure goals.
Dose & Administration:
Initial Therapy: The usual starting dose of Olmesartan Medoxomil/Amlodipine Besilate is 20/5 mg
one tablet once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum dose of 40/10 mg once daily as needed to control blood pressure. This combination may be taken with or without food. This combination may be administered with other antihypertensive agents.
Initial therapy with this combination products is not recommended in patients >75 years old or with hepatic impairment.
Replacement therapy: Olmesartan Medoxomil/Amlodipine Besilate may be substituted for its individually titrated components. When substituting for individual components, the dose of one or both of the components can be increased if blood pressure control has not been satisfactory.
Add-on Therapy: Olmesartan Medoxomil/Amlodipine Besilate may be used to provide additional blood pressure lowering for patients not adequately controlled with Amlodipine (or another dihydropyridine Calcium Channel Blocker) alone or with Olmesartan Medoxomil (or another angiotensin II receptor blocker) alone or as directed by the physician.
Clascoterone
Composition:
Clascon cream: Each gram cream contains Clascoterone INN 10 mg.
Indication:
Clascon cream is indicated for the topical treatment of acne vulgaris in patients of 12 years of age and older.
Dose & Administration:
Clean the affected area gently then apply a thin uniform layer of Clascon cream twice per day in the morning and evening to the affected area. Avoid accidental transfer of Clascon cream into eyes, mouth or other mucous membranes. If contact with mucous membranes occurs, this should be rinsed thoroughly with water or as directed by the physician.
Empagliflozin + Metformin
Composition
Glifomet Tablet: Each film coated tablet contains Empagliflozin INN 5 mg and Metformin Hydrochloride BP 500 mg.
Indication
Glifomet is indicated as an adjunct to diet and exercise to improve glycemic control in adults with
type 2 diabetes mellitus when treatment with both Empagliflozin and Metformin Hydrochloride is
inappropriate. Empagliflozin is indicated to reduce the risk of cardiovascular death in adults with
type 2 diabetes mellitus and established cardiovascular disease.
Dose & Administration
• The starting dose of Glifomet should be based on the patient's current regimen.
• Take twice daily with meals, with gradual dose escalation to reduce the gastrointestinal
side effects due to Metformin Hydrochloride.
• The maximum recommended dose is 12.5 mg empagliflozin /1000 mg metformin
hydrochloride twice daily.
• Assess renal function before initiating Glifomet. Do not initiate or continue Glifomet if creatinine
levels are greater than or equal to 1.5 mg /dL for males or 1.4 mg /dL for females or if eGFR
is below 45 mL /min /1.73 m2or as directed by the physician.
Ozenoxacin
Composition:
Zenocin Cream: Each gram cream contains Ozenoxacin INN 10 mg.
Indication:
Zenocin Cream is indicated for the topical treatment of impetigo due to Staphylococcus aureus or Streptococcus pyogenes in adult and paediatric patients 2 months of age and older.
Dose & Administration:
Apply a thin layer of Zenocin cream topically to the affected area twice daily for five days or as directed by the physicians.
Omeprazole
Composition
Ometac® 20: Each capsule contains Omeprazole BP 20 mg.
Indications:
Ometac®is indicated for the treatment of gastroesophageal reflux disease including reflux esophagitis, acid reflux disease, duodenal and benign gastric ulcers, Helicobacter pylori eradication in peptic ulcer disease, prophylaxis of acid aspiration, Zollinger-Ellison Syndrome and for the treatment of NSAID-associated gastric ulcers, duodenal ulcers or gastroduodenal erosions.
Dosage & Administration:
Reflux esophagitis: The usual dose is 20 mg once daily for 4 weeks or as directed by the physician.
Zollinger-Ellison Syndrome: Recommended initial dose is 60 mg once daily which is to be adjusted individually. Doses above 80 mg should be given in two divided doses.
Duodenal & Benign Gastric Ulcer: The usual dose is 20 mg once daily. The majority of patients with duodenal ulcer are healed after 4 weeks. For gastric ulcer, the treatment should be continued up to 8 weeks.
Elderly: No doses adjustment is required.
Children: No experience in using the drug in children.
Amorolfine
Composition:
Curafin Cream: Each gram cream contains Amorolfine Hydrochloride BP equivalent to Amorolfine 2.5 mg.
Curafin Cream is indicated for the treatment of Tinea pedis (athlete's foot), Tinea cruris, Tinea inguinalis, Tinea corporis, Tinea manuum & Pityriasis versicolor.
Dose & Administration:
To be applied to affected skin areas once daily following cleansing (in the evening). The treatment should be continued without interruption until clinical cure, and for 3 -5 days thereafter. The required duration of treatment depends on the species of fungi and on the localization of the infection. In general, treatment should be continued for at least two to three weeks. With foot mycoses, up to six weeks of therapy may be necessary or as directed by the physicians.
Metformine
Composition:
NVmet™ 500 Tablet: Each film coated tablet contains Metformin Hydrochloride BP 500 mg.
NVmet™ 850 Tablet: Each film coated tablet contains Metformin Hydrochloride BP 850 mg.
NVmet™ 500 SR Tablet: Each sustained release tablet contains Metformin Hydrochloride BP 500 mg.
Indication:
NVmet™, as monotherapy, is indicated as an adjunct to diet and exercise to improve glycemic control in patients with type 2 diabetes. NVmet™ is also indicated for use in combination with other agents, that do not result in adequate glycemic control.
Dose and administration:
Diabetes mellitus: Adult and child over 10 years initially 500 mg with breakfast for at least 1 week, then 500 mg with breakfast and evening meal for at least 1 week, then 500 mg with breakfast, lunch and evening meal; usual max. 2 g daily in divided doses.
Polycystic ovary syndrome: initially 500 mg with breakfast for 1 week, then 500 mg with breakfast and evening meal for 1 week, then 1.5-1.7 g daily in 2-3 divided doses or as directed by the physician.
Gliclazide
Composition:
Glix 80 Tablet: Each Tablet contains Gliclazide BP 80 mg.
Glix 60 MR Tablet: Each modified release tablet contains Gliclazide BP 60 mg.
Indication:
Indicated for the treatment of Type-2 Diabetes.
Dose & Administration:
Glix 80 Tablet: Initially 40-80 mg daily, adjusted according to response, increased if necessary up to 160 mg once daily. Dose to be taken with breakfast, dosage higher than 160 mg to be given in
divided doses; maximum 320 mg per day.
Glix MR Tablet: Initially 30 mg daily, dose to be taken with breakfast, adjust dose according to response every 4 weeks (after 2 weeks if no decrease in blood glucose); maximum 120 mg per day or as directed by the physician.
Linagliptin + Metformin
Composition
Diplin M 500 Tablet: Each film coated tablet contains Linagliptin INN 2.5 mg & Metformin Hydrochloride BP 500 mg.
Indications
Diplin M 500 is a dipeptidyl peptidase-4 (DPP-4) inhibitor and biguanide combination product indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both Linagliptin and Metformin is appropriate.
Dose & Administration
The maximum recommended dose is Linagliptin 2.5 mg/Metformin Hydrochloride 1000 mg twice daily or as directed by the physician.
Linagliptin
Composition:
Diplin 5 Tablet: Each film coated tablet contains Linagliptin INN 5 mg.
Indication:
Diplin is indicated as an adjunct to diet and exercise to improve glycemic control in patients with type 2 diabetes mellitus.
Dose & Administration:
The recommended dose of Diplin is 5 mg once daily. No dosage adjustment is required for hepatic or kidney impaired patients or as directed by the physician.
Nortripline
Composition:
Nortin™ 10 Capsule: Each capsule contains Nortriptyline Hydrochloride BP equivalent to 10 mg Nortriptyline.
Nortin™ 25 Capsule: Each capsule contains Nortriptyline Hydrochloride BP equivalent to 25 mg Nortriptyline.
Indication:
Depressive illness, Panic disorder & Neuropathic pain.
Dosage & administration:
Depression, low dose initially increased as necessary to 75-100 mg daily in divided doses or as a single dose (max. 150 mg daily);
Adolescent and Elderly patient : 30- 50 mg daily in divided doses. Neuropathic pain, initially 10 mg daily at night, gradually increased if
necessary to 75 mg daily; higher doses under specialist supervision. Or as directed by the physician
Flupentixol+ Melitracen
Composition
Meltix Tablet: Each film coated tablet contains Flupentixol Hydrochloride BP equivalent to 0.5 mg Flupentixol
Indications
It is indicated in various types of anxiety, depression and apathy. These include-
• Psychogenic depression
• Depressive neurosis
• Masked depression
• Psychosomatic affections accompanied by anxiety and apathy
• Menopausal depression
Dosage & Administration
Adults: Usually 2 tablets daily at morning and noon. In severe cases the morning dose may be increased to 2 tablets.
Elderly patients: 1 tablet in the morning.
Maintenance dose: Usually 1 tablet in the morning. In cases of insomnia or severe restlessness additional treatment with a sedative is recommended in acute phase or as directed by the physician.
Clonazepam
Composition
Conpan ODT 0.5 : Each orally disintegrating tablet contains Clonazepam USP 0.5 mg.
Conpan ODT 1 : Each orally disintegrating tablet contains Clonazepam USP 1 mg.
Conpan ODT 2 : Each orally disintegrating tablet contains Clonazepam USP 2 mg.
Indications
Clonazepam is indicated in all forms of epilepsy, status epilepticus, infantile spasms,
myoclonic seizures, akinetic and atonic seizures, partial seizures, bipolar affective
disorder, panic attacks etc.
Dose & Administration
The standard dose of clonazepam must be individually adjusted according to the patient's
clinical response and tolerance of the drug. As a general rule, Clonazepam is started with
low single dose in a new patient. Adults: 1mg (elderly, 0.5 mg), initially at night for 4
nights, increased according to response over 2-4 weeks to usual maintenance dose of 4-8
mg which is usually given at night as a single dose or may be given in 3-4 divided doses if
necessary. Children: Up to 1 year, initially 0.25 mg, increased as above to usual
maintenance dose of 0.5-1 mg, 1-5 years initially 0.25 mg, increased as above to 1-3 mg,
5-12 years, initially 0.5 mg, increased as above to 3-6 mg.
Direction for administration of Conpan Orally Disintegrating Tablet:
This tablet must be handled with dry hand. The tablet to be placed on tongue and let it be
dissolved. It should not be chewed, broken, or crushed. Like other conventional oral
tablets, it also can be swallowed with a glass of water or as directed by the physician.
Clonidine
Composition
Clonipres 0.1 Tablet: Each tablet contains Clonidine Hydrochloride USP 0.1 mg.
Clonipres ER 0.1 Tablet: Each Extended release tablet contains Clonidine Hydrochloride USP 0.1 mg.
Indication
Clonidine is indicated in the treatment of hypertension. It may be employed alone or concomitantly with other anti-hypertensive agent. Clonidine Hydrochloride is also indicated for the treatment of menopausal flushing, opioid withdrawal & alcohol withdrawal syndrome. Clonidine Hydrochloride extended release tablet is indicated for the treatment of attention deficit
hyperactivity disorder (ADHD) as monotherapy.
Dosage & Administration
Adult: The dose of Clonidine must be adjusted according to the patient's individual blood pressure response.
Initial dose: 0.1 mg twice a day (morning & bed time). Elderly patients may be benefited from a lower initial dose.
Maintenance dose: Further increments of 0.1 mg per day may be made at weekly intervals if necessary, until the desired result is achieved. The therapeutic dose can be given from 0.2 mg to 0.6 mg per day in divided doses. In renal impairment, dose of Clonidine Hydrochloride must be
adjusted according to the degree of impairment & patients should be monitored carefully.
Attention deficit hyperactivity disorder : Clonidine IR 5 mcg/kg/day or Clonidine ER 0.1 mg/day for 8 weeks.
Menopausal flushing : 0.1 mg - 0.4 mg daily
Alcohol withdrawal : 0.3 - 0.6 mg every 6 hourly.
It can be taken orally with food or without food or as directed by the physician.
Cefaclor
Composition:
Navacef™ Paediatric Drops (15 ml): Each 1.25 ml contains Cefaclor Monohydrate USP equivalent to 125 mg of Cefaclor.
Indication
Respiratory Tract Infections: Pneumonia, bronchitis, pharyngitis, tonsillitis, otitis media.
Urinary Tract Infections: Pylonephritis, cystitis etc.
Skin & Soft Tissue Infections: Impetigo, pyoderma, cellulitis, pruritis etc.
Dosage & Administration
Adults: 250 mg three times daily (Dose should be doubled in severe cases, max. 4 g daily). Children (over 1 month): 20 mg/kg/day in three divided doses (Dose should be doubled in severe cases, max 1 g daily). 1-5 years: 125 mg three times daily.
Over 5 years: 250 mg three times daily, (Dose should be doubled in severe cases) or as directed by the physician.
Flucloxacillin
Composition:
Flubiotic 250 Capsule: Each capsule contains flucloxacillin sodium BP equivalent to 250 mg
flucloxacillin.
Flubiotic 500 Capsule: Each capsule contains flucloxacillin sodium BP equivalent to 500 mg
flucloxacillin.
Indications:
Flucloxacillin is indicated for the treatment of infections due to gram positive organisms (including beta-lactamase producing staphylococci).
Typical Indications
*Skin and soft tissue infections:
Boils, abscesses, carbuncles, furunculosis, cellulitis, infected skin conditions, for example, ulcer eczema and acne, infected wounds, infected burns, protection for skin graft, otitis media, externa & impetigo.
* Respiratory tract infections:
Pneumonia, lung abscess, empyema, sinusitis, pharyngitis, tonsilitis, quinsy.
* Other infections caused by flucloxacillin-sensitive organisms:
Osteomyelitis, enteritis, urinary tract infection, meningitis, septicaemia. Flucloxacillin is also indicated for the use as a prophylactic agent during major surgical procedures where appropriate, for example cardiothoracic and orthopaedic surgery.
Dosage and Administration:
Oral dosage should be administered one hour before meal or two hour after meal.
Usual adult dose (including elderly patients): Oral 250 mg four times daily. Dosage may be doubled in severe infections.
Osteomyelitis, endocarditis: Up to 8 gm daily in divided doses (6 to 8 hourly) or as directed by the physician.
Cefuroxime
Composition
FixcefTM 250 Tablet: Each film coated tablet contains Cefuroxime Axetil BP equivalent to Cefuroxime 250 mg.
FixcefTM 500 Tablet: Each film coated tablet contains Cefuroxime Axetil BP equivalent to Cefuroxime 500 mg.
FixcefTM Powder for Suspension: After reconstitution each 5 ml suspension contains Cefuroxime Axetil BP equivalent to Cefuroxime 125 mg.
Indication
Pharyngitis/tonsillitis, Acute bacterial otitis media, Acute bacterial maxillary sinusitis, Lower respiratory tract infections including pneumonia, Acute bacterial exacerbations of chronic bronchitis and secondary bacterial infections of acute bronchitis, Skin and Skin-Structure Infections, Bone and Joint Infections, Gonorrhea, Early Lyme disease, Septicemia, Meningitis, Surgical Prophylaxis.
Dosage & Administration
Oral
INFECTIONS DOSAGE DURATION
Tablet (May be administered without regard to meals)
Adolescents & adults(13 years & above)
Pharyngitis or Tonsillitis 250 mg twice daily 5 - 10 days
Acute bacterial maxillary sinusitis 250 mg twice daily 10 days
Acute bacterial exacerbation of chronic bronchitis 250 - 500 mg twice daily 10 days
Secondary bacterial infections of acute bronchitis 250 - 500 mg twice daily 5 - 10 days
Uncomplicated skin & skin-structure infections 250 - 500 mg twice daily 10 days
Uncomplicated urinary tract infection 125 - 250 mg twice daily 7 - 10 days
Uncomplicated gonorrhea 1000 mg single dose - - -
Lyme disease 500 mg twice daily 20 days
Paediatric patients (Upto12 years)
Acute otitis media 250 mg twice daily 10 days
Acute bacterial maxillary sinusitis 250 mg twice daily 10 days
Suspension
Paediatric patients (3 months to 12 years)
Pharyngitis or Tonsillitis 20 mg/kg/day in two divided doses 5-10 days
Acute otitis media 30 mg/kg/day in two divided doses 10 days
Acute bacterial maxillary sinusitis 30 mg/kg/day in two divided doses 10 days
or as directed by the physicians.
Esomeprazole
Composition
Esotac 20 Capsule: Each delayed release capsule contains Esomeprazole Magnesium Trihydrate
USP equivalent to Esomeprazole 20 mg.
Esotac 40 Capsule: Each delayed release capsule contains Esomeprazole Magnesium Trihydrate
USP equivalent to Esomeprazole 40 mg.
Esotac 20 Tablet: Each enteric coated tablet contains Esomeprazole Magnesium Trihydrate USP
equivalent to Esomeprazole 20 mg.
Esotac 40 Tablet: Each enteric coated tablet contains Esomeprazole Magnesium Trihydrate USP
equivalent to Esomeprazole 40 mg.
Indication
1. Healing of erosive esophagitis
2. Long-term management of esophagitis
3. Symptomatic gastroesophageal reflux disease
4. H. pylori eradication for the treatment of duodenal ulcer (Triple therapy with Esomeprazole,
clarithromycin and amoxicillin)
Dose and Administration
Healing of erosive Esophagitis: 20 mg or 40 mg once daily for 4-8 weeks. For those patients who
have not healed after 4-8 weeks of treatment, an additional 4-8 weeks course of Esomeprazole may be considered.
Long-term management of esophagitis: 20 mg once daily.
Symptomatic gastroesophageal reflux disease: 20 mg once daily for 4 weeks.
H. pylori eradication for the treatment of duodenal ulcer (Triple therapy): 20 mg Esomeprazole once daily with 500 mg clarithromycin twice daily and 1g amoxicillin twice daily for 7-10 days or as directed by the physician.
Cefuroxime + Clavulanic Acid
Composition
Fixcef™ Plus 250 Tablet: Each film coated tablet contains Cefuroxime Axetil BP equivalent to Cefuroxime 250 mg and Diluted Potassium Clavulanate BP equivalent to Clavulanic Acid 62.5 mg.
Fixcef™ Plus 500 Tablet: Each film coated tablet contains Cefuroxime Axetil BP equivalent to Cefuroxime 500 mg and Diluted Potassium Clavulanate BP equivalent to Clavulanic Acid 125 mg.
Indication
Pharyngitis/tonsillitis, Acute bacterial otitis media, Acute bacterial maxillary sinusitis. Acute bacterial exacerbations of chronic bronchitis and secondary bacterial infection of acute bronchitis, Uncomplicated skin and skin-structure infections, Uncomplicated urinary tractinfections, Uncomplicated gonorrhoea (urethral and endocervical), Early lyme disease (erythema migrans).
Dosage & Administration
For oral administration
Fixcef™ Plus Tablet: The usual course of v therapy with Cefuroxime-Clavulanic acid tablets is 5 to 7 days for treatment of bronchitis and 7 to 10 days for other infections.
Adolescents and Adults (13 years and older)
Infection Dosage Duration (days)
Pharyngitis/tonsillitis 250 mg b.i.d. 05-10
Acute bacterial maxillary sinusitis 250 mg b.i.d. 10
Acute bacterial exacerbations of chronic bronchitis 250 or 500 mg b.i.d. 10
Secondary bacterial infections of acute bronchitis 250 or 500 mg bid. 05-10
Uncomplicated skin and skin-structure infections 250 or 500 mg b.i.d. 10
Uncomplicated urinary tract infections 250 mg b.i.d. 07-10
Uncomplicated gonorrhoea 1,000 mg Single dose
Early Lyme disease 500 mg bid. 20
Paediatric Patients (who can swallow tablets whole)
Infection Dosage Duration (days)
Acute otitis media 250 mg b.i.d. 10
Acute bacterial maxillary sinusitis 250 mg b.i.d 10
or as directed by the physician.
Polyethylene Glycol + Propylene Glycol
Composition
Syscol Sterile Eye Drops: Each ml drops contains Polyethylene Glycol 400 USP 4 mg & Propylene Glycol BP 3 mg.
Indications
Syscol Sterile Eye Drops is indicated for the temporary relief of burning and irritation due to dryness of the eye.
Dosage and administration
Instill 1 drop 4 times daily in the affected eye (s) or as needed or as directed by the physician.
Bilastine
Composition
Allertin 20 Tablet: Each tablet contains Bilastine INN 20 mg.
Allertin Oral Solution: Each ml oral solution contains Bilastine INN 2.5 mg.
Indication
Seasonal Allergic Rhinitis: Bilastine is indicated for the symptomatic relief of nasal and non-nasal symptoms of seasonal allergic rhinitis (SAR).
Chronic Spontaneous Urticaria: Bilastine is indicated for the relief of the symptoms associated with chronic spontaneous urticaria (CSU) (e.g. pruritus and hives).
Dosage & Administration
Allertin 20 Tablet: Adults and adolescents 12 years of age and above: 20 mg tablet once daily. The tablet should be taken one hour before or two hours after intake of food.
Allertin Oral Solution: 4 ml of oral solution once daily for children 4 to 11 years of age. Recently, it has been approved in Latin America for children from 2 years of age. The oral solution should
be taken one hour before or two hours after intake of food or fruit juice or as directed by the physician.
Sulconazole
Composition:
Sulderm Cream: Each gram cream contains Sulconazole Nitrate USP 10 mg.
Indication:
Sulderm Cream is indicated for the treatment of Tinea Pedis (athlete's foot), Tinea Cruris, Tinea Corporis & Pityriasis Versicolor.
Dose & Administration:
A small amount of cream should be gently massaged into the affected and surrounding skin areas once or twice daily, in case of Tinea Pedis where administration should be twice daily. Early relief of symptoms is experienced by the majority of patients and clinical improvement may be seen fairly soon after treatment is begun; however, Tinea Corporis, Tinea Cruris and Tinea Versicolor should be treated for 3 weeks and Tinea Pedis for 4 weeks to reduce the possibility of recurrence. Or as directed by the physician.
Alogliptin
Composition
Aloglip 12.5 Tablet: Each film coated tablet contains Alogliptin Benzoate INN equivalent to Alogliptin 12.5 mg.
Aloglip 25 Tablet: Each film coated tablet contains Alogliptin Benzoate INN equivalent to Alogliptin 25 mg.
Indication
Aloglip is indicated as an adjunct to diet and exercise to improve glycaemic control in adults with type-2 diabetes mellitus.
Dose & Administration
The recommended dose in patients with normal renal function or mild renal impairment is 25 mg once daily. The dose for patients with moderate renal impairment is 12.5 mg once daily with or without food or as directed by the physicians.
Azithromycin
Composition:
Azirox™ 500 Tablet: Each film coated tablet contains Azithromycin Dihydrate USP equivalent to Azithromycin 500 mg. Azirox™ Powder for Suspension (20 ml, 35 ml & 50 ml): Each 5 ml reconstituted suspension contains Azithromycin Dihydrate USP equivalent to Azithromycin 200 mg.
Indication:
Acute bacterial exacerbations of chronic obstructive pulmonary disease, Community-acquired pneumonia, Acute otitis media, Sinusitis, Pharyngitis, Tonsillitis, Skin and soft tissue infections, Urethritis and Cervicitis, Genital ulcer disease, Sexually transmitted diseases (STD), Typhoid fever.
Dosage and Administration:
Adult dose: For Acute bacterial exacerbations of chronic obstructive pulmonary disease, community-acquired pneumonia, sinusitis, pharyngitis, tonsillitis and Skin and Soft tissue infections due to the indicated organisms: 500 mg once daily orally for 3 days or as an alternative given over 5 days with 500 mg dose on the first day followed by 250 mg once daily on days 2 through 5. Genital ulcer disease, non-gonococcal urethritis and cervicitis: 1 g (1000 mg) as a single dose. Urethritis and cervicitis due to Neisseria Gonorrhoeae: 2g (2000 mg) as a single dose. Sexually transmitted diseases: 1 g as a single dose. Typhoid fever: 500 mg once daily for 7 days. Pediatric dose: For children over 6 months recommended dose is 10mg/kg once daily for 3 days 15-25 kg: 200 mg once daily for 3 days, 26-35 kg: 300 mg once daily for 3 days, 36-45 kg : 400 mg once daily for 3 days, Azirox™ should be taken at least 1 hour before or 2 hour after meal or as directed by the physician.
Cetrizine
Composition:
Trizin™ Sterile Eye Drops: Each ml contains Cetirizine
Dihydrochloride BP equivalent to Cetirizine 2.40 mg.
Preservative: Sodium Perborate BP 0.001%.
Indications:
Trizin™ Sterile Eye Drop is indicated for the treatment of
ocular itching associated with allergic conjunctivitis.
Dosage and administration:
The recommended dosage of Trizin™ Sterile Eye Drop is
to instill one drop in each affected eye twice daily
(approximately 8 hours apart) or directed by the
physician.
Nepafenac
Composition:
Neparact™ TS Sterile Eye Suspension: Each ml contains Nepafenac INN 3 mg.
Preservative: Sodium Perborate BP 0.001%
Indications:
Neparact™ TS Sterile Eye Suspension is indicated for-
The treatment of post-operative ocular pain and
inflammation including cataract surgery.
Dosage & administration:
•Instill 1 drop once daily 1 day prior to cataract surgery
and continued on the day of surgery and through the
first 2 weeks of the post-operative period.
•An additional drop should be administered 30 to 120
minutes prior to surgery.
Hypromellose
Composition
Iclear Sterile Eye Drops: Each ml drops contains Hypromellose BP 3 mg.
Indications
Hypromellose is used to treat dry eye conditions where reduced or absent of normal lachrymal
secretion. Lachrymal secretions (tears) are produced to keep the eye lubricated and clean.
Dosage and Administration
Therapy of dry eye syndrome requires an individual dosage regimen. The usual dose for Iclear is 1 drop into the conjunctival sac 3 to 5 times per day or as directed by the physician or as directed by the physician.
Moxifloxacin
Composition
Cinagen™ Sterile Eye Drops: Each ml contains Moxifloxacin Hydrochloride BP equivalent to Moxifloxacin 5 mg.
Cinagen™ XG Sterile Eye Drops: Each ml contains Moxifloxacin
Hydrochloride BP equivalent to Moxifloxacin 5 mg (enriched with Xanthan Gum BP 0.4%).
Indications
Cinagen™ is indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of following organisms:
Aerobic gram-positive microorganism: Corynebacterium species, Micrococus luteus, Staphylococcus aureus, Staphylococus epidermis, Staphylococus haemolyticus, Staphylococcus hominis, Staphylococcus warneri, Streptococcus pneumoniae, Streptococcus viridans group Aerobic Gram-negative microorganisms: Acinetobacter iwoffi, Haemophilusinfluenzae, Haemophilus parainfluenzae.
Dosage and administration
Cinagen™ Sterile Eye Drops: One drop in the affected eyes 3 times per day for 7 days or as directed by the physician.
Cinagen™ XG Sterile Eye Drops: One drop in the affected eyes 2 times per day for 7 days or as directed by the physician.
Carboxymethylcellulose
Cfresh TM Liquigel Sterile Eye Drops: Each ml drops contains Carboxymethylcellulose Sodium
Indications
It is indicated for use as a lubricant in dry eye (keratoconjunctivitis sicca) including relief of
burning, irritation and/or discomfort due to dryness of the eye.
Dosage & Administration
Instill 1 or 2 drops in the affected eye(s) or as directed by the physician.
Glycerin + Hypromellose + Polyethylene glycol + Tetrahydrozoline + Zinc Sulfate
Composition:
Artiforte™ Sterile Eye Drops: Each ml contains Glycerin
USP 2 mg, Hypromellose BP 3.6 mg, Polyethylene glycol
400 USP 10 mg, Tetrahydrozoline Hydrochloride USP 0.5
mg, Zinc Sulfate Monohydrate USP equivalent to Zinc
Sulfate 2.5 mg. Preservative: Sodium Perborate BP 0.001%.
Indications:
Artiforte™ is indicated for the relief of discomfort and
redness of the eye due to minor eye irritations, for relief of
dryness of the eye, for the temporary relief of burning and
irritation due to exposure to wind or sun, for protection
against further irritation.
Dosage and Administration:
1 to 2 drops in the affected eye(s) up to 4 times daily or as
directed by the physician.
Children under 6 years of age: as per suggestion of
physician or as directed by the physician
Montelukast
Composition:
Arokast™10: Each film coated tablet contains Montelukast Sodium USP equivalent to Montelukast 10 mg.
Arokast™ 4 FT: Each film coated tablet contains Montelukast Sodium USP equivalent to Montelukast 4 mg.
Arokast™ 5 FT: Each film coated tablet contains Montelukast Sodium USP equivalent to Montelukast 5 mg.
Indication:
Montelukast is indicated for the prophylaxis of asthma and management of chronic asthma; symptomatic relief of seasonal allergic rhinitis in patients with asthma.
Dosage & Administration:
Prophylaxis of asthma:
Adult and Child over 15 years: 10 mg once daily in the evening.
Child 6-15 years: 5 mg once daily in the evening.
Child 6 months-6 years: 4 mg once daily in the evening.
Seasonal allergic rhinitis:
Adult and Child over 15 years, 10 mg once daily in the evening. The safety and efficacy of Montelukast was demonstrated in clinical trials where it was administered in the evening without
regard to the time of food ingestion.
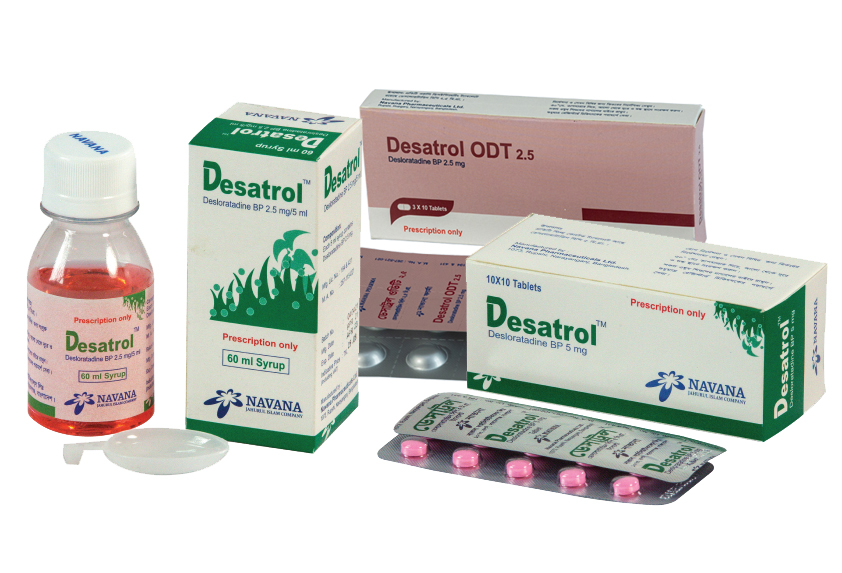
Desloratadine
COMPOSITION :
Desatrol™Tablet : Each film coated tablet contains Desloratadine BP 5 mg.
Desatrol™Syrup : Each 5 ml syrup contains Desloratadine BP 2.5 mg.
INDICATION :
Allergic Rhinitis: Desatrol™ is indicated for the relief of the nasal and nonnasal symptoms of allergic rhinitis (seasonal and perennial).
Chronic idiopathic Urticaria: Desatrol™ is indicated for the symptomatic relief of pruritus, reduction in the number of hives and size of hives, in patients with chronic idiopathic urticaria.
DOSE AND ADMINISTRATION :
Tablet : In adults and children 12 years of age and over, the recommended dose is 5 mg once daily.
In patients with liver or renal impairment, a starting dose of one 5 mg tablet every other day is recommended.
Syrup : Children 2-5 years of age : 2.5 ml once daily, 6-11 years of age : 5 ml once daily. Adults and children 12 years of age and over : 10 ml once daily. It may be administered with or without food. Or as directed by the physician.
Fexofenadine
Composition:
Odafen™ 120: Each film coated tablet contains Fexofenadine
Hydrochloride USP 120 mg.
Odafen™ 180: Each film coated tablet contains Fexofenadine
Hydrochloride USP 180 mg.
Odafen™ Suspension: Each 5 ml suspension contains Fexofenadine Hydrochloride USP 30 mg.
Indication:
Seasonal Allergic Rhinitis:
Odafen™ tablet are indicated for the relief of symptoms
associated with seasonal allergic rhinitis in adults and children 6 years of age and older. Odafen™ oral suspension is indicated for the relief of symptoms associated with seasonal allergic rhinitis in children 2 to 11 years of age. Symptoms to treat
effectively:
sneezing, rhinorrhea, itchy nose/palate/throat, itchy/watery/red eyes.
Chronic Idiopathic Urticaria:
Odafen™ of Chronic Idiopathic Urticaria in adults and children 6 years of age and
older.
Odafen™ manifestations of Chronic Idiopathic Urticaria in children 6 months to 11 years
of age. Fexofenadine Hydrochloride significantly reduces pruritus and the number
of wheals.
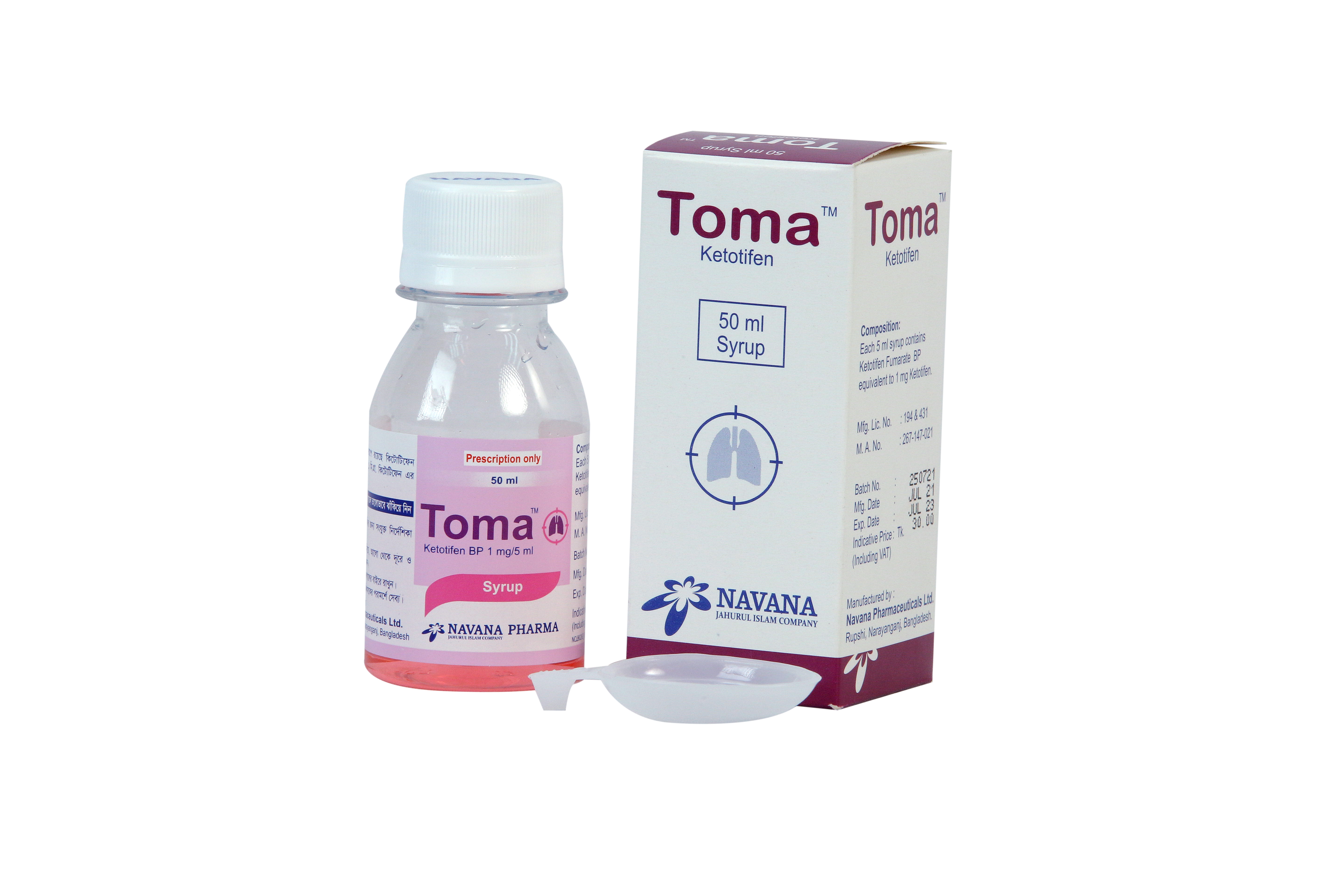
Ketotifen
Composition
Toma™ Tablet: Each tablet contains Ketotifen Fumarate BP equivalent to 1 mg Ketotifen.
Toma™ Syrup: Each 5 ml syrup contains Ketotifen Fumarate BP equivalent to 1mg Ketotifen
Indication
• Prophylactic treatment of bronchial asthma.
• Allergic rhinitis ( seasonal and perennial).
• Allergic conjunctivitis.
• Allergic conditions such as hay fever, itchy rash.
Dose & Administration
Adult: 1 mg twice daily with food. If necessary the dose may be increased to 2mg twice daily in severe cases.
Children above 2 years: 1 mg twice daily with food.
From 6 month to 2 years: 0.05 mg (0.25 ml) per kg of body weight twice daily.
Use in elderly: Same as adult dose.
Missed dose: If a dose is missed, should be taken as soon as possible. If it is almost time for the next dose, skip the missed dose and the regular dosing schedule should be maintained. Dose should not be doubled in case of a missed dose.
Patient's known to be easily sedated should begin treatment with 0.5 to 1 mg at night for the first few days or as directed by the physician
Hyoscine Hydrobromide
Composition:
Joytrip 150: Each chewable tablet contains Hyoscine Hydrobromide BP 150 mcg.
Joytrip 300: Each tablet contains Hyoscine Hydrobromide BP 300 mcg.
Indication:
Prevention and control of nausea and vomiting associated with motion sickness/travel sickness.
Dosage & Administration:
Ideally, take tablets 30 minutes before journey. Adults and children over 13 years: 300 mcg (Maximum 900 mcg in 24 hours). Children age 7-12 years: 150-300 mcg (Maximum 300 mcg in 24 hours). Children age 4-7 years: 150 mcg (Maximum 300 mcg in 24 hours). Children age 3-4 years: 75 mcg (Maximum 150 mcg in 24 hours). Not recommended under 3 years or as directed by the physician.
Dexlansoprazole
Composition
Dextac™ 30 Capsule: Each capsule contains Dexlansoprazole INN 30 mg as enteric coated pellets.
Dextac™ 60 Capsule: Each capsule contains Dexlansoprazole INN 60 mg as enteric coated pellets.
Indications
• Healing of Erosive Esophagitis: Dextac™ (Dexlansoprazole) is indicated for healing of all grades of Erosive Esophagitis (EE) for up to 8 weeks.
• Maintenance of Healed Erosive Esophagitis: Dextac™ (Dexlansoprazole) is indicated to maintain healing of EE and relief of heartburn for up to 6 months.
• Symptomatic Non-Erosive Gastroesophageal Reflux Disease: Dextac™ (Dexlansoprazole) is indicated for the treatment of heartburn associated with symptomatic Non Erosive Gastroesophageal Reflux Disease (GERD) for upto 4 weeks.
Dosage & Administration
Dextac™ (Dexlansoprazole) dosing recommendations
Indication Dose Frequency
Maintenance of Healed EE and relief of heartburn 30 mg Once daily
Symptomatic Non-Erosive GERD 30 mg Once daily for 4 weeks
Healing of EE 60 mg Once daily for 8 weeks
Important Administration Information
• Dextac™ (Dexlansoprazole) can be taken without regard to food or the timing of food.
• Dextac™ (Dexlansoprazole) should be swallowed whole.
Alternatively, Dextac™ (Dexlansoprazole) capsules can be administered as follows:
- Open capsule.
- Sprinkle intact granules on one table spoon.
- Swallow immediately. Granules should not be chewed.
Missed Dose: If a capsule is missed at its usual time, it should be taken as soon as possible. But
if it is too close to the time of the next dose, only the prescribed dose should be taken at
appointed time. A double dose should not be taken. or as directed by the physicians.
Pregabalin
Composition
Pregan ER 82.5: Each extended release tablet contains Pregabalin BP 82.5 mg.
Pregan ER 165: Each extended release tablet contains Pregabalin BP 165 mg.
Indications
• Diabetic peripheral neuropathy
• Post-herpetic neuralgia
• Menopausal depression
Dosage & Administration
The recommended dose is Once daily. Pregabalin ER tablet should be administered once daily after an evening meal. It should be swallowed whole and should not be split, crushed or chewed.
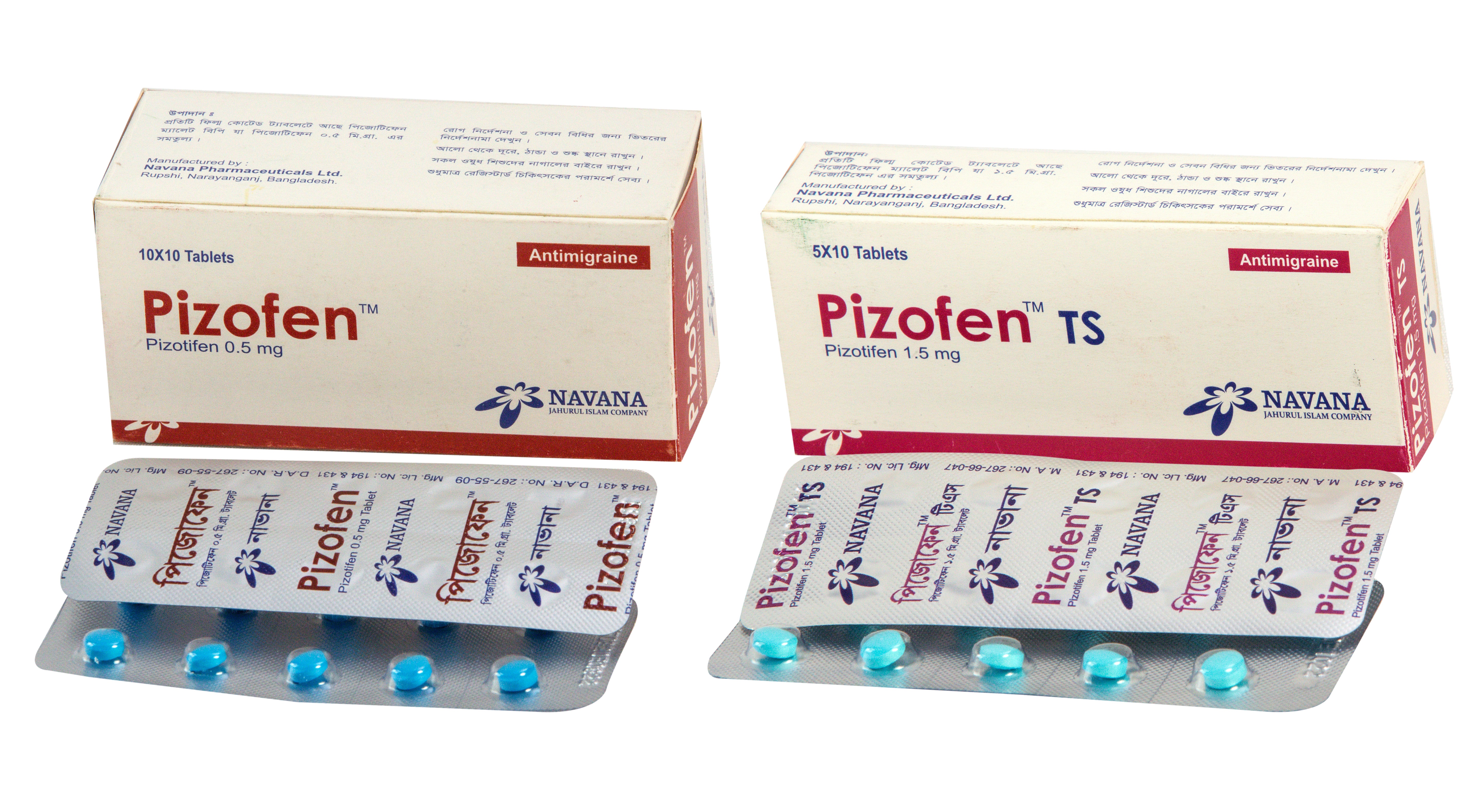
Pizotifen
COMPOSITION :
Pizofen™ Tablet : Each film coated tablet contains Pizotifen
malate BP equivalent to Pizotifen 0.5 mg
Pizofen™ TS Tablet : Each film coated tablet contains Pizotifen
malate BP equivalent to Pizotifen 1.5 mg
INDICATION :
It is used as prophylactic treatment of vascular headaches of the migraine types, such as classic migraine, common migraine and cluster headache.
DOSE AND ADMINISTRATION :
Adult : Usually 1.5 mg daily, which may be taken as a single
dose at night or in three divided doses. Dose should be adjusted to individual patient's requirements. Upto a maximum of 4.5 mg may be given as a single dose daily.
Children : Dose is 1 mg as a single dose at night using 0.5 mg
tablet. Maximum dose is upto 1.5 mg daily usually in divided
dose.
Or as directed by the physician.
Flunarizine
Composition
Imigra™ 5 Tablet: Each film coated tablet contains Flunarizine Dihydrochloride BP equivalent to 5 mg Flunarizine.
Imigra™ 10 Tablet: Each film coated tablet contains Flunarizine Dihydrochloride BP equivalent to 10 mg Flunarizine.
Imigra™ 5 Capsule: Each capsule contains Flunarizine Dihydrochloride BP equivalent to 5 mg Flunarizine.
Imigra™ 10 Capsule: Each capsule contains Flunarizine Dihydrochloride BP equivalent to 10 mg Flunarizine
Indication
O Symptomatic treatment of vestibular vertigo & dizziness.
O Peripheral vascular diseases (Intermittent claudication, Raynaud's phenomenon, paresthesia, cold extremities etc.).
O Epilepsy resistant to conventional anti-epileptic therapy.
O Prophylaxis of classic (with aura) or common (without aura) migraine.
Dose and Administration
For vertigo: The recommended maximum daily dose of Flunarizine in the treatment of vertigo is 10 mg daily in adults and 5 mg daily in children (<40 kg).
For epilepsy: An optimal therapeutic dosage in epileptic patients receiving other anti-epileptic drug is 15 mg to 20 mg daily in adults and 5 to 10 mg daily in children.
For migraine prophylaxis: Starting dose is 10 mg daily at night for adult patients less than 65 years of age and 5 mg daily for patients older than 65 years.
Maintenance treatment: If a patient's response is satisfactory and if a maintenance treatment is needed, the dose should be decreased. The patient should have 5 days treatment in a week at the same daily dose and 2 successive drug free days. Treatment should be stopped after 6 months and reinitiated only if the patient relapses.
Or as directed by the physician
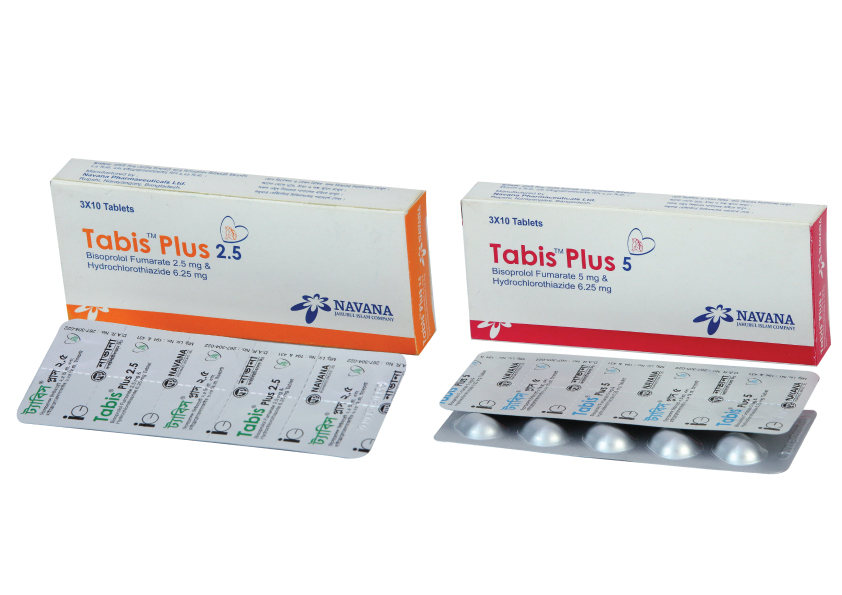
Bisoprolol + Hydroclorothiazide
Composition:
Tabis™ Plus 2.5 Tablet: Each film coated tablet contains Bisoprolol Fumarate USP 2.5 mg and Hydrochlorothiazide BP 6.25 mg.
Tabis™ Plus 5 Tablet: Each film coated tablet contains Bisoprolol Fumarate USP 5 mg and Hydrochlorothiazide BP 6.25 mg.
Indication:
Tabis™ Plus is indicated for the management of hypertension.
Dose & Administration:
The initial dose is 2.5/6.25 mg once daily. Subsequent titration (14 day intervals) may be carried out with Tabis™ Plus tablets up to the maximum recommended dose 20/12.5 mg once daily as appropriate.
Bisoprolol
Composition
Tabis 2.5 Tablet: Each film coated tablet contains Bisoprolol Fumarate USP 2.5 mg.
Tabis 5 Tablet: Each film coated tablet contains Bisoprolol Fumarate USP 5 mg.
Indications
• Hypertension
• Angina
• Adjunct in stable moderate to severe heart failure.
Dosage & Administration
Hypertension: The dose of Bisoprolol must be individualized to the needs of the patient. The usual starting dose is 5 mg once daily. In some patients, 2.5 mg may be an appropriate starting dose. If the antihypertensive effect of 5 mg is inadequate, the dose may be increased to 10 mg and then, if necessary, to 20 mg once daily.
Angina: Usually 10 mg once daily (5 mg may be adequate in some patients) max 20 mg daily.
Heart Failure: Initially 1.25 mg once daily (in the morning) for 1 week then, if well tolerated, increased to 2.5 mg once daily for 1 week, then 3.75 mg once daily for 1 week, then 5 mg once daily for 4 weeks, then 7.5 mg once daily for 4 weeks, then 10 mg once daily, max 10 mg daily or as directed by the physician.
Olmesartan + Hydrochlorothiazide
Composition:
Olmeben™ Plus 20/12.5 Tablet: Each film coated tablet contains Olmesartan Medoxomil USP 20 mg & Hydrochlorothiazide BP 12.5 mg
Indication:
It is indicated for the treatment of hypertension. This fixed dose combination is not indicated for initial therapy.
Dose & Administration:
Hypertension
The usual starting dose of Olmeben™ Plus 20/12.5 is one tablet once daily. Dosing should be
individualized. Depending on the blood pressure response, the dose may be titrated at intervals of
2-4 weeks.
Patients with Renal Impairment:
The usual regimens of therapy with Olmeben™ Plus 20/12.5 may be followed provided the patient's creatinine clearance is >30 mL/min. In patients with more severe renal impairment, loop diuretics are preferred to thiazides. So, Olmeben™ Plus 20/12.5 is not recommended. Patients with Hepatic Impairment: No dosage adjustment is necessary with hepatic impairment.
Or as directed by the physician.
Olmesartan
Composition:
Olmeben™ 20 Tablet: Each film coated tablet contains Olmesartan Medoxomil USP 20 mg.
Olmeben™ 40 Tablet: Each film coated tablet contains Olmesartan Medoxomil USP 40 mg.
Indication:
It is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.
Dose & Administration:
The usual starting dose of Olmesartan is 20 mg once daily. Dosing should be individualized. Depending on the blood pressure response, the dose may be increased after 2 weeks to 40 mg. Olmesartan may be administered with or without food. No initial dosage adjustment is recommended for elderly patients, for patients with moderate to marked renal impairment (creatinine clearance < 40 ml/min) or with moderate to marked hepatic dysfunction. If blood pressure is not controlled by Olmesartan alone, a diuretic may be added. Olmsartan may be administerd with other antihypertensive agents. Or as directed by the physician.
Clopidogrel + Aspirin
Composition:
Navix™ Plus Tablet: Each film coated tablet contains Clopidogrel Bisulfate USP equivalent to Clopidogrel 75 mg and Aspirin BP 75 mg.
Indication:
The preparation is used for the prevention of ischemic events, myocardial infarction, stroke and cardiovascular death in patients with acute coronary syndrome.
Dosage & Administration:
The recommended dose is once daily or as directed by the physician.
Clopidogrel
Composition:
Navix™ Tablet: Each film coated tablet contains Clopidogrel Bisulfate USP equivalent to 75 mg Clopidogrel.
Indication:
Clopidogrel is indicated for the reduction of atherosclerotic events (myocardial infarction, stroke and vascular death) and in patient with atherosclerosis documented recent stroke, recent myocardial infarction or established peripheral arterial disease (PAD).
Dosage & Administration:
The recommended dose of Clopidogrel is 75 mg once daily with or without food. No dosage adjustment is required for elderly patients or patients with renal disease or as directed by the physician.
Levamlodipine
Composition
L-Amlo 1.25 Tablet: Each tablet contains Levamlodipine Maleate INN equivalent to Levamlodipine 1.25 mg.
L-Amlo 2.5 Tablet: Each tablet contains Levamlodipine Maleate INN equivalent to Levamlodipine 2.5 mg.
L-Amlo 5 Tablet: Each tablet contains Levamlodipine Maleate INN equivalent to Levamlodipine 5 mg.
Indication
L-Amlo is a calcium channel blocker which is used alone or in combination with other antihypertensive agents for the treatment of hypertension. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions.
Dosage & Administration
* Adult recommended dose: 2.5 mg orally once daily with maximum dose 5 mg once daily. For small, fragile or elderly patients or patients with hepatic insufficiency, 1.25 mg once daily starting dose is recommended.
* Paediatric starting dose: 1.25 mg to 2.5 mg once daily or as directed by the physicians.
Naftifine
Composition:
Nafgal Cream: Each gram cream contains Naftifine Hydrochloride USP 20 mg.
Indications:
Nafgal Cream is indicated for the treatment of interdigital tinea pedis, tinea cruris and tinea corporis caused by the organism Trichophyton rubrum.
Dosage & Administration:
Apply a thin layer of Nafgal Cream once-daily to the affected areas plus half inch margin of healthy surrounding skin for 2 weeks or as directed by the physician.
Luliconazole
Composition:
Lulider Cream: Each gram cream contains Luliconazole INN 10 mg.
Indications:
Lulider Cream is indicated for the topical treatment of interdigital tinea pedis, tinea cruris and tinea corporis, in patients 2 years of age and older.
Dosage & Administration:
Tinea pedis: A thin layer of cream should be applied to the affected area and approximately 1 inch of the immediate surrounding area(s) once daily for two weeks.
Tinea cruris and tinea corporis: A thin layer of cream, should be applied to the affected area and approximately 1 inch of the immediate surrounding area(s) once daily for one week or as directed by the physician.
Ceftriaxone
Composition:
Topcef™ 250 mg IM Injection: Each vial contains dry substance equivalent to 250 mg Ceftriaxone (as sterile Ceftriaxone Sodium BP) with 2 ml Lidocaine Hydrochloride BP 1% Injection.
Topcef™ 500 mg IM/IV Injection: Each vial contains dry substance equivalent to 500 mg Ceftriaxone (as sterile Ceftriaxone Sodium BP) with 2 ml Lidocaine Hydrochloride BP 1% Injection for IM injection or 5 ml Water for Injection BP for IV injection.
Topcef™ 1 g IM/IV Injection: Each vial contains dry substance
equivalent to 1 g Ceftriaxone (as sterile Ceftriaxone Sodium BP) with
3.5 ml Lidocaine Hydrochloride BP 1% Injection for IM injection or 10
ml Water for Injection BP for IV injection.
Topcef™ 2 g IV Injection: Each vial contains dry substance equivalent
to 2 g Ceftriaxone (as sterile Ceftriaxone Sodium BP) accompanied by
2 ampoules each contain 10 ml water for injection.
Indication:
Topcef™ is indicated in sepsis, meningitis, neurosyphillis, abdominal infections (peritonitis, infections of the biliary and gastrointestinal tracts), infections of the bones, joints, soft tissue, skin and of wounds, renal and urinary tract infections, respiratory tract infections, ear, nose and throat infections, genital infections, including gonorrhoea, perioperative prophylaxis of infections.
Dosage and Administration:
Adults and children over 12 years: The usual dose is 1-2 g of Topcef™ once daily (every 24 h). In severe cases, the dose may be raised to 4 g once daily. Neonates, infants and children up to 12 years: Neonates (up to 14 days): A daily dose of 20-50 mg/kg body weight, not to exceed 50 mg/kg. Infants and children (15 days to 12 years): A daily dose of 20-80 mg/kg. For children with body weights of 50 kg or more, the usual adult dose should be used. Elderly patients: The dosage recommended for adults require no modification in case of geriatric patients. Duration of therapy: The usual duration of therapy is 4 to 14 days; in complicated infections, longer therapy may be required. When treating infections caused by Streptococcus pyogenes, therapy should be continued for at least 10 days. Meningitis: In bacterial meningitis in infants and children, treatment begins with dose of 100 mg/kg (not to exceed 4 g) once daily. Gonorrhoea: a single IM dose of 250 mg Topcef™ is recommended. Perioperative prophylaxis: a single dose of 1-2 g depending on the risk of infection of 30-90 minutes prior to surgery. In colorectal surgery, administration of Topcef™ with or without a 5-nitroimidazole, e.g. Omidazole, has been proven effective.
Impaired renal and hepatic function: In patients with impaired renal function, there is no need to reduce the dosage of Topcef™ provided hepatic function is intact. In patients with liver damage, there is no need for the dosage to be reduced provided renal function is intact Or as directed by the physician.
Cefixime
Composition:
Duracef™ 200 Capsule: Each capsule contains Cefixime Trihydrate BP equivalent to Cefixime 200 mg.
Duracef™ 400 Capsule: Each capsule contains Cefixime Trihydrate BP equivalent to Cefixime 400 mg.
Duracef™ DS PFS (50 ml): Each 5 ml reconstituted suspension contains Cefixime Trihydrate BP equivalent to Cefixime 200 mg.
Duracef™ PFS (50 ml): Each 5 ml reconstituted suspension contains Cefixime Trihydrate BP equivalent to Cefixime 100 mg.
Duracef™ PFS (30 ml): Each 5 ml reconstituted suspension contains Cefixime Trihydrate BP equivalent to Cefixime 100 mg.
Duracef™ Paediatric Drops (21 ml): Each ml reconstituted suspension contains Cefixime Trihydrate BP equivalent to Cefixime 25 mg.
Duracef™ Max PFS (10 ml): Each 5 ml reconstituted suspension contains Cefixime Trihydrate BP equivalent to Cefixime 500 mg.
Duracef™ Max PFS (20 ml): Each 5 ml reconstituted suspension contains Cefixime Trihydrate BP equivalent to Cefixime 500 mg.
Indications:
Otitis media- caused by Streptococcus pneumoniae (including Penicillinase resistant strains), Streptococcus pyogenes, Haemophilus influenzae (including beta-lactamase producing strains), Moraxella catarrhalis (including beta-lactamase producing strains). Pharyngitis/Tonsillitis- caused by Streptococcus pyogenes. Uncomplicated Urinary Tract infection- Caused by E. coli, Proteus mirabilis. Uncomplicated Gonorrhoea- Caused by N. gonorrhoeae (including Penicillinase & non-Penicillinase resistant strains).
Dosage and Administration:
Adults: The recommended dose is given as a single dose of 400 mg or 200 mg twice daily for 7-14 days according to the severity of infections. Uncomplicated Gonorrhoea: 400 mg single dose.
Children:> 6 months: 8 mg /kg/ day in 1-2 divided doses, 6 months-1 year: 75 mg daily, 1-4 years: 100 mg daily, 5-10 years: 200 mg daily, > 10 years: As same as adult dose or as directed by the physician
Olopatadine
Composition:
Alleloc DS Sterile Eye Drops: Each ml drops contains Olopatadine Hydrochloride USP equivalent to Olopatadine 2 mg.
Indications:
Allergic conjunctivitis.
Dosage and administration:
Alleloc DS: Instill one drop in the affected eye (s) once daily or as directed by the physician.